In 2023, there were an estimated 1.5 million animal species on Earth– only one is truly blue.
The Obrina Olivewing butterfly is the only observed animal that internally produces a blue pigment; the scales of other blue butterflies are complex structures that only refract blue light.
But blue’s rarity is not limited to the organic world.
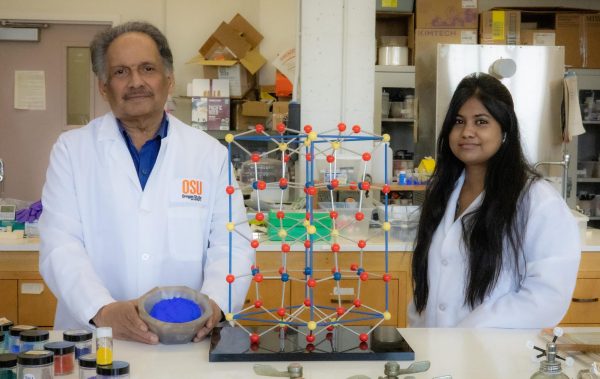
“For 200 years people have been looking for a new blue color pigment,”said Mas Subramanian, a Distinguished Professor in Oregon State University’s Department of Chemistry. “We made it unexpectedly.”
The last blue pigment to come onto the market was in the 1930s, with the discovery of Phthalo blue. Before that, only a handful of other pigments were available to artists– most of them either toxic, faded quickly, or expensive.
Ultramarine blue was so coveted, the famous Dutch artist Vermeer went bankrupt trying to purchase it.
“In 2008, we started a project trying to make a material which is kind of an interesting magnetic and dielectric ferroelectric material… We combine those two and then we try to create a material called multiferroic,” Subramanian said.
In other words, Subramanian and his team weren’t looking to find a new pigment.

The discovery came when Indium oxide and Manganese oxide were combined, with the intention to make one of these multiferroic materials. “And finally, when we made this solid solution, suddenly we saw this beautiful blue come out of the blue,” Subramanian said.
Subramanian recognized the rarity of blues from his time in private industry, and put a patent on the new pigment with the name YInMn blue, after its primary components: Yttrium (Y), Indium (In), and Manganese (Mn). It is now commercially available, and has made it into many books and paintings, some found in Subramanian’s office.
“Yeah, it’s very bright, very brilliant,” Subramanian said.
Pigment research is now a huge program in his lab. Anjali Verma, a graduate student who started working there in December of 2021, came upon the research about as accidentally as YInMn blue came to be. “I saw Mas giving a talk and then I was like, ‘this is interesting.’”
In order to understand the draw of YInMn blue, we must look down to the molecular world.
Photons – pieces of energy that translate into light – traversing through the air collide with atoms on the surface of a pigment.
If the atom’s energy matches the photon’s energy, the atom absorbs that photon and the light that corresponds with it.
If the light is absorbed by the pigment atoms, we are unable to see it. But if the light’s energy does not match the atom’s energy, it bounces back and is absorbed by our eyes.
“(YInMn) absorbs energy in the orange region, which gives rise to this beautiful blue color,” Subramanian said.
What really sets YInMn blue apart, though, is its ability to form crystal lattices.
A crystal lattice is the structure atoms make when many bind together– some may look like a simple chain-link fence, while others can make complex cage-looking structures. If one were to peer down into the molecular structure of YInMn, they’d see a diamond-looking structure.
To Subramanian and his researchers, this structure was unexpected. Indium, Magnesium, and Yttrium don’t usually arrange themselves in this way.
But, like its blue cousins, YInMn can be costly to make.
The element Indium is expensive and drives up the cost of the pigment. To create more pigments, Subramanian and his team began swapping in more inexpensive elements.
Now, the hallway connecting the various Subramanian lab rooms are lined with colorful posters depicting the rainbow of hues the research has been able to produce. “It has become a pretty big program in our lab,” Subramanian said.
The research has extended far outside the confines of the lab, though.
“That is because it touches not only the scientific world, but also the art world, the architectural world,” Subramanian said.
He has received letters from private companies, other researchers and laboratories, as well as prisoners and first graders interested in the work of the Subramanian lab.
“Say that I found a new superconductor, yes, it’s exciting, very exciting, right? But not to a person who sits in a prison, who may not understand all the quantum mechanics behind it.” Subramanian said, referring to the visual nature of this research and how it can appeal to everyone.
But one color is still missing from the rainbow.
“One color we could not make so far is red… we’re still trying to make something to replace a toxic Cadmium Red, Vermillion.” Subramanian said. The lab recently received a grant from the National Science Foundation to fund the search.
“There are so many pigments available for red but they contain lead, mercury– and they all are toxic, right?” Verma said. The pigment industry has been looking for a healthy, environmentally-friendly red pigment for quite some time.
Creating a new pigment, as it turns out, is quite a bit harder than it sounds– especially since so little is known about the chemistry behind color in the first place.
“Most of the discoveries come from shooting in the dark,” Subramanian said, “We always explain the color mechanisms after we discover something, right? We don’t go the other way.”
Predicting a color is difficult, Subramanian explained, in part because of the complexity of color in the first place. Many interacting properties are responsible for creating color, almost as many as there are for destroying it.
A very small deviation in bond distances can change a mineral from red to green.
“You know rubies and emeralds? On both of them, the chromophore– which gives color– in ruby, it’s chromium three plus iron, and in emeralds, it’s also chromium three-plus,” Verma said. “So see, how come that color is entirely different? It’s just that bond distances on the structure are different in both of them.”
(Jiritana Tungkawachara, OMN Photographer)
But there are dozens of other tiny interactions happening on the molecular level that can make just as much of an impact.
“Even with all that advancement in computing and calculations, it’s not easy to punch in something in the computer and say, ‘give me the red color,’” Subramanian said.
Subramanian’s labs, full of x-ray machines, open lab notebooks with margin-to-margin calculations, and crucibles of colorful pellets are impressive. It makes it hard to believe that there are still so many unknowns in the world of color.
But Subramanian says that’s the best part.
“I think, as a scientist – I’ve been doing this for 40-plus years – I’ll tell you this, there’s no other better profession I can find,” Subramanian said.
Even with only a few years of experience, Verma agrees that the unknown is oftentimes the most fascinating part of research. “If it doesn’t come out the right way you feel frustrated, right? It is for me. But every time I feel frustrated, I feel excited too. If I don’t find something new, I’m like, ‘Okay, I’m going to try this (another way)’.”
The world of color still has its secrets for now. But after years of research and discoveries, Subramanian and his team are curious as ever: “It’s a never-ending frontier.”